When one writes of "fine glass" one usually means glass that has a significant amount of lead oxide in its composition, although it is acknowledged that non-lead glass, when carefully made, can also be described as "fine glass". In this latter category are Venetian cristallo, of soda and lime, and Bohemian crystal that uses potash and lime. In this GUIDE it is fine glass with a high lead content with which we are concerned, excepting a few references to Bohemian cut and engraved glass of the mid-nineteenth century. Elville (1960, p. 25) describes lead glass as follows:
As lead is a very heavy substance, it considerably increases the density of the glass, enhancing its power to refract and disperse the light transmitted through it more than any other glass-making material. It is this property of density and corresponding power to disperse light that gives to lead crystal glass its unrivalled sparkle and brilliancy when cut, and its bell-like note when sharply struck. . . .Elville might also have added the property of "softness" that lead glass has because it is this characteristic that makes the glass cutter's blank so amenable to the cutting process. Before discussing the various formulae that have been used to produce lead glass, or glass-of-lead as it was often called in the past, especially in England, a few basic definitions should be mentioned.
Although Elville writes of "lead crystal glass" he could just as well have written "lead glass". The key word is "lead" not "crystal". The latter term is a flexible one and has more than one meaning. Lead content, on the other hand, is more or less rigidly defined, based on international agreement. For example, new glass sold today with a lead content of at least 30% (by weight) can be called "full lead" crystal while glass with a lead content less than 30%, but 24% or more, can be called "half lead" crystal (Newman 1977, p. 83). In practice, however, the lead glass found at one's department store will likely be labeled as "full lead crystal" or "24% lead crystal". "Half lead" is not a good marketing term (note 1)!
The word "crystal" is also often used to describe glass that contains no lead oxide at all. This applies to today's glass as well as to glass made in the past. "Crystal" is probably best thought of as meaning colorless glass with no lead content unless the latter is explicitly stated. Confusingly, however, one can also find "colored crystal" which may or may not contain lead oxide but which is definitely not colorless! As a result of all this, the glass described by Elville -- and that which we hold in high regard -- should always be called lead glass. not crystal. And never "flint glass". "Flint" is simply an old term that refers to the silica content of all glass, not to the lead content of lead glass, although it has been used in this way in the past. It is a term, likely crystal, that has been much abused.
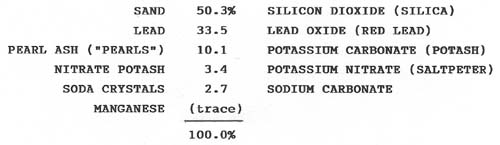
Because one often reads about the "secrets of the glasshouse", including the formulae for making various kinds of glass, it comes as a pleasant surprise to discover that several formulae, or recipes, for lead glass are actually readily available. The formula given here was used by the Dithridge Flint Glass Works of New Brighton, PA and recorded by Edward Dithridge, Jr. on 24 Sep 1890. It is one of three formulae for blanks that are described as "Flint Glass rich and heavy for cutting". A note attached to this formula states: "This is virtually the flint or crystal glass of France and England as used for their fine ware both for heavy cut ware and light blown ware including the most delicate." (Innis 1976, pp. 483-484). Incidentally, the Dithridge glassworks also included a cutting shop where the company produced a variety of patterns that it advertised as "rich cut" (Innis 1976, p. 455).
In the following table the ingredients to the left of the percentage-amounts are those as described by Dithridge; alternative descriptions have been added for clarification on the right. Pearl ash, nitrate potash, and soda are fluxing agents used to lower the temperature required to consolidate, or fuse, the ingredients in the mixture or batch. They are collectively known as "salts". Nitrate potash is also called niter. Manganese is a decolorizer. The percentages are based on a total batch weight of 2980 lbs.

Samples of the three main ingredients in lead glass, from the left: sand, red lead, and potash (Image: Grehan 1981, p. 61).

The other two Dithridge formulae for lead glass modify the above recipe, the simpler of the two having the so-called classic ratio of 3 : 2 : 1, exactly, for sand, lead, and "salts" (consisting entirely of potash). In percentage terms this ratio becomes 50%, 33.3%, and 16.7%, the simplest possible lead-potash glass. This is the ratio developed by the Englishman George Ravenscroft and his successor, Hawley Bishop, in the late seventeenth century (note 2). Considered to be "Britain's greatest contribution to the world of glass" (Woodward 1984, p. 66), this basic formula has persisted, unchanged except for small amounts of additional chemicals, through the centuries. It was used in Ireland during the Anglo-Irish period of cut glass, c1780-c1825 (Westropp 1978, p. 175), and Wilson (1994, p. 46) quotes it as being used by Apsley Pellatt in England during the first half of the nineteenth century.
In this country the classic ratio was the one recommended by Henry Rowe Schoolcraft, writing in 1812 (Daniel 1950, p. 46), and probably used by him for a brief period, 1815-1817, at the Keene (Marlboro Street) Glass Works, Keene, NH (Wilson 1972, p. 160). It was also the ratio used by Deming Jarves at the Boston & Sandwich Glass Company (Swan 1986, p. 13), 1825-1888, and by William Gillinder at his company, from 1861 (formula not yet confirmed). Elville emphasizes that the percentage of lead oxide in lead glass has remained remarkably constant since the late seventeeth century:
. . . [T]he lead content of . . . glass has not been less than 30 per cent since the potash-lead formula was established in the Ravenscroft period. Moreover, very little change occurred in the content of lead during the whole of the eighteenth century, nor, indeed, during the one that followed, or up to the present. The greatest variation . . . does not represent more than 5 per cent of lead (Elville 1960, pp. 259-260).The formulae used by Schoolcraft, Jarves, Gillinder, and Dithridge indicate that Elville's observation is also valid on this side of the Atlantic, at least through the nineteenth century. An average percentage of lead oxide of 33.6% is calculated for these glasshouses, with a range of 4.6%, from 32.0% to 36.6%.
Formulae are lacking for the twentieth century with one exception: that used by Frederick Carder for colorless lead glass at the Steuben Glass Works. He lowered the lead content from the "industry average" of 33.6% to 28.4% while silica was increased to 53.5%. "Salts" also increased slightly to 17.4% (note 3).
Sinclaire and Spillman (1977, p. 198) state that Steuben lead glass was "unremarkable" at this time (1904-1918), and they report that T. G. Hawkes testified in 1904 that the factory used "a great deal less lead than the glass manufactured by the Corning Glass Works." Lead content at C. Dorflinger & Sons and Libbey would also seem to have been less than average, if the figure of "around 28%" lead oxide cited by Sinclaire represents typical conditions at these factories during the early twentieth century. This figure also applies to Dorflinger glass during the 1890s when "best batch" had the following composition: sand (49.6%), lead (28.9%), potassium carbonate (19.0%), and potassium nitrate (2.5%) according to Louis Dorflinger's diary (Barbe and Reed, 2003, p. 85) (note 4).
The only other figures we have for percentage of lead oxide are for present-day factories: about 33% at Waterford (Grehan 1981, p. 60) and 36% at Edinburgh Crystal (Woodward 1984, p. 66). It would be interesting to undertake a more comprehensive study, comparing the foregoing figures and formulae with those at the major glasshouses that were operating during the American brilliant period of cut glass, but apparently this was one area where the glasshouses of the day kept their "secrets" under wraps.
NOTES:
1. It is interesting to note that in his investigative report on fraudulent cut glass -- near-copies that were cut on modern blanks and were sold as authentic, antique glass during the 1980s -- Ian Berke notes that the modern blanks were tested by the Corning Glass Laboratories. They found that the lead content of the modern blanks "was quite uniform, 24-25% by weight" ("Problems in Cut Glass", Maine Antique Digest, Mar 1990, p. 28A).
2. The story of the discovery of lead glass is told in detail by Charleston (1984) against a background of commercial intrigue and the political, religious, and social conditions of Restoration England with its revival of the spirit of scientific inquiry.
3. The ingredients in the Carder formula add up to only 99.81% which is too great a difference from 100% to be due to "rounding error". The Carder formula was gradually replaced by a new formula called G10M at Steuben about 1932. It involved "decreasing the normal impurity levels of batch materials thereby improving the glass's ability to transmit ultraviolet radiation, adding brilliance to the glassware. The new Steuben crystal is . . . 'still essentially the composition as introduced by Mr. Carder at the start of his venture in Corning. But the important small subtraction and addition as noted [none are provided here], have given the attraction (sic) and durable features. . . .'" (memo dated 14 Jun 1966, as quoted in Dimitroff 1998, page n.a.). Presumably the G10M formula, with a lead-oxide percentage of about 28%, is still in use today.
4. Sinclaire also reports that the lead content in blanks from "the great European [glass]houses . . . hovered around 35% lead content" at this time. She is of the opinion that "in pieces of similar size and thickness, the 25% higher lead content [i.e., the increase from 28% to 35%] is easy to feel." (see Sinclaire, Estelle, 1988: The Belgian connection, The Hobstar, Vol. 10, No. 6, p. 5, Mar).
The writer has handled many examples of Dorflinger cut glass and has never been conscious of any significant reduction in the lead-oxide content of its products relative to that produced by contemporary companies. The only situation where reduced levels of lead oxide may have been present in the glass he has handled occurred with "figured blanks" as used by companies other than Dorflinger (who never used "figured blanks") after about 1905. This "cut glass" often seemed lighter than normal. Typically, an eight-inch berry bowl would weight about one-half pound lighter than a cut pattern that used a plain blank. But this is only anecdotal evidence, and somewhat subjective at that.
Updated 3 May 2004